
 |
| DEPARTMENT OF MECHANICAL ENGINEERING |
| Laboratory of Optical and Fluidic Technologies Home | Research | Members | Openings | News | Publications | Links | Contact |
Visit Other Labs
← → |
OPTICAL DIAGNOSTIC TECHNIQUES FOR MICRO/NANOFLUIDICS
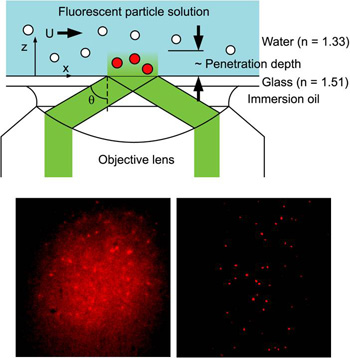
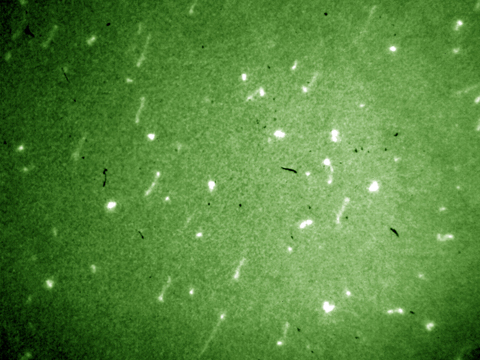
Tracer particle-based image velocimetry is a widely used technique to visualize flow field in the macroscopic scale. Optical microscopy and microscale particle image velocimetry (µPIV) are insufficient for nanoscale investigations due to large optical resolutions (~0.5 µm) and their limit on two-dimensional imaging with restricted depth-resolving power. Recently Dr. Huang developed a novel evanescent wave-based imaging technique termed “three-dimensional total internal reflection velocimetry” (3D-TIRV) for conducting three-dimensional fluidic and colloidal measurements with a 10-nm displacement resolution. In 3D-TIRV, fluorescent tracer particles within 1 µm from a two-medium interface are illuminated with an evanescent field. Under this configuration, the emission intensities of the fluorescent particles decay exponentially as a function of distance to the interface. Thus the in-plane displacement of a tracer particle can be tracked with standard particle tracking velocimetry while its out-of-image-plane motion can be tracked with its intensity. An evanescent wave microscope has recently been completed by our research group inside the Analytical Diagnostics Laboratory (ADL) of the Binghamton University Center for Excellence in Small Scale Systems Integration and Packaging, and is currently being utilized in a wide variety of micro/nanofluidic research areas.
|

|
NEAR-WALL FLUIDIC AND COLLOIDAL DYNAMICS
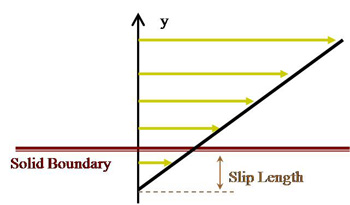
Apparent Slip at the Liquid Interfaces
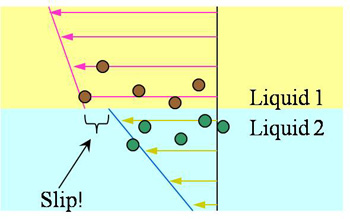
The no-slip boundary condition at a fluid/solid interface has been an “empirical law” until being challenged in the past decade. Our previous experimental studies have shown that a finite slip length, though small, can exist at an interface between an aqueous solution and a sub-nanometer smooth solid surface. Such a slip can be further enhanced if the surface is rendered hydrophobic (chemically incompatible). Such findings also raise questions on another empirical law that has been widely considered valid: the continuity boundary condition at the interface of two liquids. Molecular dynamics simulations by Koplik and Banavar (PRL, 2006) have shown that an “apparent velocity slip” can occur between two immiscible liquids while no direct experimental studies have been conducted. We are currently applying the 3D-TIRV system to measure velocities in the vicinity of the interface in both liquid phases. Specially designed PDMS microfluidic channels are fabricated to flow the immiscible liquids side-by-side and one on top of the other while direct velocity measurements are conducted.
|
|
Colloidal Dynamics of a Near-Wall Particle
The theory of translational and rotational dynamics of a near-wall spherical particle in a shear flow had been reported by Goldman, Cox and Brenner in 1967. Near-wall particles are expected to experience increases in both translational and rotational drags, but the decreased translational mobility of a near-wall particle in a shear flow has only been demonstrated recently by using 3D-TIRV. We are currently expanding the capability of our 3D-TIRV system to making direct measurement of near-wall particle rotation both in shear flow and in quiescent fluid.
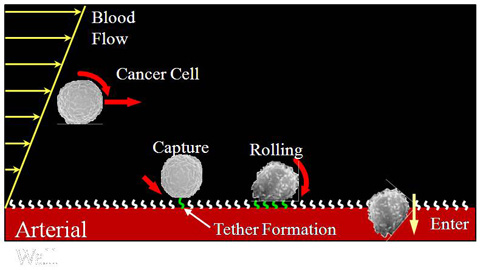
Adhesion Dynamics of Circulating Cells
When leukocytes are needed at an infected area to fight off invading microorganisms, leukocytes that come into contact with blood vessel walls upstream of the infection site are loosely tethered onto the blood vessel wall cell lining and subsequently “roll” along the vessel surface before finding an opening and entering the infected tissue. When cancer cells start to metastasize through the circulatory system, they are believed to attach to a remote organ by the same mechanism (i.e. tethering and rolling before entering the secondary sites). Experimental studies have shown that cell tethering and adhesion only occurs in flow above a certain critical shear. A postulated explanation is that shear-induced rotation increases the cell surface areas sampling the blood vessel wall and thus significantly improves the probability of ligand-receptor chemical bond formation between the cells and blood vessel surfaces. Previously reported experimental studies, however, were not capable of making direct rotational measurements and compared cell adhesion behaviors in shear flow with that in quiescent fluid only, and not with the behaviors in a plug (non-shear) flow. Therefore, the roles of shear and cell rotation in adhesion still lack experimental confirmation and remain speculative. Recently, ligand-coated micron-sized particles and selectin-coated glass surfaces were demonstrated to capture the biochemical and rolling behaviors critical to leukocyte adhesion. Using the same colloidal model in microfluidic channels to mimic cells in blood vessels and the 3D-TIRV, our group directly measure the translation and rotation of the colloidal cell models in pressure-driven (shear) and electro-osmotic (non-shear) flows.
|
SINGLE-MOLECULE STRUCTURE AND INTERACTION DYNAMICS
In the past decades, experimental studies of macromolecules such as DNA and proteins have greatly enhanced our understanding of the dynamic behaviors of these basic units for life. A thorough grasp of their mechanical behaviors will empower researchers with the knowledge to develop methods of manipulation that mimics the effectiveness and efficiency that nature has perfected over millions of years of evolution. The most significant studies of molecular mechanics reported in literature had employed atomic force microscope, optical tweezers and magnetic tweezers as force spectroscopes to examine molecular elastic properties. These methods, however, suffer two significant drawbacks that prevent investigation into the true macromolecular physics. First, most of the reported experimental methods can only apply a linear stretching force and measure the only linear extension of a macromolecule. Second, all of the methods mentioned above require rigidly holding both ends of a macromolecule. Thus these experimental setups arbitrarily impose constraints on the position of the applied force and the direction of stretching. These inflexibilities limit scientists to formulate physical models of molecular mechanics based on tension-extension behaviors while ignoring the molecular torsional mechanics which allows macromolecules to naturally twist, fold and roll up. Our research group is currently developing a new optics-based force and torque spectroscopy measurement technique that will overcome the pitfalls of other methods and therefore offer opportunities to improve our understanding of single molecule dynamics. Results from this novel method of measurement are expected to lead to breakthrough developments in single molecule dynamics models.
|
MICROORGANISM DYNAMICS AND MANIPULATION
Investigation into the motion and coordination dynamics of microorganisms and their incorporation into microelectromechanical systems (MEMS) is another thrust area of research that our research group is currently pursuing. We use microfluidic, optical, and biological control means to inhibit their growth, manipulate their motions, and guide them into work production. To explore this new front of research, our research group has developed collaboration relationships with Drexel University, the Naval Undersea Warfare Center and the Binghamton Biofilm Research Center.
| |